New Hair Growth Cosmetic: Medipost Korea
Today, I am pleased to be sharing news of a stem-cell derived hair growth cosmetic product which could be available to consumers by the end of this year. While researching in the Follicle Thought lab recently 😉 I discovered the company Medipost, which is based in South Korea. Medipost is a biotechnology company that is focused on developing cord-blood derived stem cell therapeutics. Cord blood stem cells are simply stem cells which are derived from umbilical cord blood. The company currently has stem cell therapeutics in development for osteoarthritis, BPD, and Alzheimer’s.
Stem Cell Derived Hair Growth Cosmetic
Medipost first announced a patent for their “Stem Cell Culture Medium For Hair Loss Treatment” in late February 2018. The company’s news release about the patent states:
“This technique involves exposing stem cells acquired from cord blood to an artificially
created catagen phase and developing a culture medium that can prevent hair loss.”
One thing that should be taken into consideration right off the bat for many readers is the fact that this product will not contain any stem cells. This product, just like all other stem cell culture media products, contains proteins or growth factors which are secreted by the stem cells while they are in liquid suspension. Only the secretions of the stem cells, the “stem cell culture medium” is used at the end. In this case, Medipost has created an environment which mimics the biology of hair loss for the stem cells to sit in. This instigates the stem cells to react and attempt to correct the situation by secreting factors which support hair growth. For more information on this subject, please browse the PDF titled “Alopecia project using MSC conditioned media” provided by the Director of Medipost Culture Media Business.
Initial Clinical Results
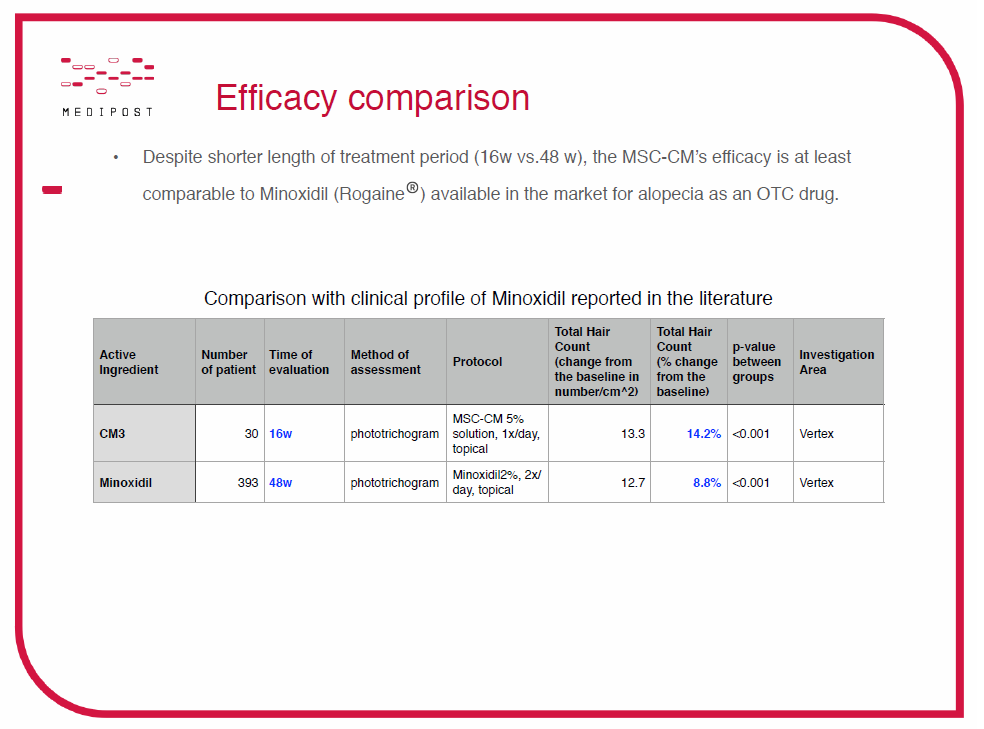
The chart above displays the comparison of clinical trials from CM-3 (Medipost’s stem cell culture medium) and minoxidil. In these trials, CM-3 was used over a period of 16 weeks and minoxidil was used over a period of 48 weeks.
I also requested some photo results from Medipost’s 30 person clinical trial of CM-3. The Director of Culture Media Business at Medipost, Jay Lee PhD, shared the following photos with me and this message: “The photo was taken not for commercial promotion purpose and you may find it not dramatic.”
I find the transparency of this company refreshing and I am intrigued by this product. For the photos above, I definitely see an improvement from baseline to week 16. Additionally, I find it odd that week 4 seems to show less hair than baseline, and that week 8 seems to show more growth than week 16. I am pretty sure these photos have been dated correctly. For the 8w and 16w comparison, perhaps the head is not tilted down enough to show the crown spot in week 8. However, this brings me back to my initial conclusion that I see an overall improvement from baseline to 16 weeks (or 4 months).
Market Release
If you haven’t had a chance to read the 4 page PDF report which is linked above, I’ll save you some time and share the details which are most important to many of us. The market launch of a CM-3 containing product, which will be sold as an OTC cosmetic product, is expected in Korea in 4Q 2018 or 1Q 2019.


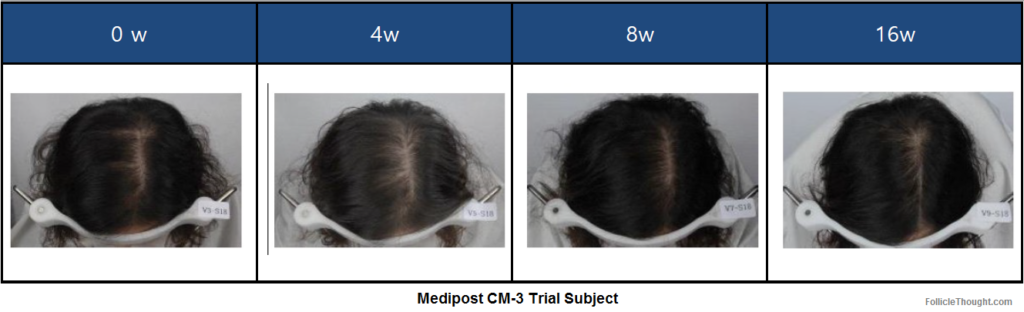
The 4 week picture being worse than the 0 week picture could well be due to shedding. This is often a frightening experience but can actually be a sign that the recipient is responding to the treatment.
Thanks for the contribution Bernhard!
The question is: will it regrow hair for a Norwood 6 or 7?
Good indeed to have a transparent company.
Regardless of the order, I think it is fair to say that there is a strenghening of the hair (even if the patient goes by different phases, I guess what matters if the result after 4 months)
I do agree with Trevor though regarding the N6 or N7. In most cases that I have seen, when products deal with proteins and others, the result is mostly positive for N1 to N3. If people from medipost are reading the comments (or if you are still chatting directly with them Admin), it could be cool to have info regarding N6 and 7 and reasonable anticipated results for them.
Thx
Thanks Ry. I can try to follow up on that in a week or so if Medipost does not see the question here.
cool thx a lot!
Thanks for agreeing to followup on the N6 or N7 growth question.
If they can show growth for N6 or N7, this product will be, absolutely a mega-hit. Also, results for N6 or N7 are much easier to see in photographs, unlike in N1 thru say N5, where we mostly have doubts when looking at pics that may have different head tilt positions, lighting, etc.
I am hoping this works for N6 or N7 as well. If it does, it will work for any and all Norwood types.
Thanks for a great find!
Thanks for your comments. I’m Jay of Medipost and is in charge of this new product development. The primary objective of the clinical study is for initial proof of concept and we have not followed up the patients in long term. The study was not gender specific, but the subjects recruited was primarily female patients (29:1 for 30 in total). The inclusion criteria was i) Ludwig >1 for females and ii) NH (Norwood-Hamilton) 2 or 2a for male patients at 20-60 age. It is hard to say at the moment whether the product is effective in male pattern alopecia with higher HN grade. Another trial is ongoing in bigger size and more strict protocol and hopefully I can update you of the outcome in the future.
Hi Jay,
Would you mind sharing the data from the singular male patient from the study?
Thanks,
Aaron
Positive news and a new approach to reduce hair loss! Keep us posted on product availability admin. Any idea if this product could be purchased online?
Not sure about that, but it seems if the product works well someone will take up the task of distributing internationally.
We need this soon!!
Medipost has a highly successful product called CARTISTEM, which is used for rebuilding cartilage (knee joint). They were approved for Phase 1 and Phase 2 trials in the US by FDA. CARTISTEM is based on Mesenchymal Stem Cells (MSCs) and the hair treatment is also based on MSCs. MSCs have been proven to be very effective without any side effects or negative immunomodulation responses.
(I have been following MSCs for over two years for indications such as arthritis, congestive heart failure, back pain, etc. with other companies). This is the first treatment I have come across that is close to market for hair loss.
I am very excited.
Thanks Trevor for the kind words. P1/2a trial for Cartistem in the US has been completed and we are in discussion with FDA for the next phase of development in the US. The product was approved and launched in Korea for knee cartilage defects by trauma or osteoarthritis and so far treated more than 7000 severe OA patients in Korea. We have two more products in clinical development for BPD (Broncho pulmonary dysplasia) with Pneumostem and AD (Alzheimer disease) with Neurostem both in Korea and the US. All our products are based in umbilical cord blood derived MSC. In parallel with MSC conditioned media as a functional cosmetics for hair loss that was introduced here, we also develop MSC therapy for the same indication as a therapeutic, not for cosmetics (though early in development stage).
Wow Jay, you’re saying you are also developing an MSC therapy for the treatment of hairloss at therapeutic level? I imagine it will be by injection. That’s big news!
What is therapeutic vs cosmetic in terms of hair loss? Please explain.
That’s a good question Trevor. It entirely depends on the regulatory system in each country. In May 2017, MFDS (Korean equilvalence of FDA) decided to expand the scope of functional cosmetics adding to conventional 3 (wrinkle, skin lightening and UV protection) 5 new efficacy claims including hair loss. Unlike regular cosmetic products, you should submit efficacy and safety (including clinical trial in human) data package for registration as a functional cosmetics in compliance with regulatory requirements, but compared to drug registration for drug, the regulatory burden is much lighter. In the meantime, however, there is much limitation, for example, on the scope of API, ROA and efficacy claim for functional cosmetics. For example, you may not use stem cell itself or inject the product. For efficacy claim, “hair growth” or “hair loss prevention” is not allowed, but only hair loss relief. I understand Japan have a similar regulatory system. Hope it answers.
Wait a minute. Will this drug help to regrow lost hair on my balding head? Or it just prevents still remaining hair from falling?
Sorry for english. Btw, this is my first comment. And thanks for this website.
Dear Sir, I’m from Medipost and may be in a good position to answer your question. It must regrow your lost hair based on our studies both in clinical and non-clinical (mode of action). However, as long as it is to be commercialized in cosmetics at least in Korea, we are not allowed to promote hair growth effect but only for prevention of remaining hair (to be exact, “relief of the hair loss”). Hope it answers.
Yes I would also like to know will this process be able to reactive “dead hair ” shafts or of will it depend on the stage of the atrophy.
Dear James, it is not clear yet whether the product works even on the regeneration of resting or dead follicles based on the ex vivo and clinical studies except it increases hair density and growth rate in given area of the vertex. However, some of the study on the MOA reveals that the product has something to do with the krox20 genes that was also introduced in this site a while ago https://folliclethought.com/hairclone-cont-krox20-discovery-weekly-thoughts-52317/. Thus, it needs further study whether the primed stem cell conditioned media could regenerate resting or dead hair.
Dear Jay Lee,
I have read your reply to the previous,
That is great news.
Can you say, will it be in as an injection into the scalp? Or Tablet?
Regards
James
It is being developed for a topical administration in a form of hair essence or tonic. The trial was done simply applying the product (in a liquid form) to the given area of the vertex topically without any penetration enhancing agent or device.
Jay, are penetration enhancing agents allowed and planned for the cosmetic product?
Yes penetration enhancer is allowed in cosmetics at least in Korea as long as its safety is confirmed and registered. We may also apply medical (or beauty) device to enhance penetration, for example, sono- or ionto- generators.
Hi Jay,
Thanks, it is so great to have someone who can finally come on these forums and just chat about their product in full transparency.
Good luck with that and even if does not impact that much people higher than NH 2a, it is already good to have something new and you can be proud of it.
If you can share the scope of the new test you are carrying (e.g. until which NH grade you will be testing, it would be great).
Looking forwards to the product launch.
Hi jay,
Can it increase density of transplanted hair?
Hello Vivek, no evidence for the transplanted hair yet, nor do we plan to in near future. Basically the product works in biological mechanism and I am not much positive if it works even the transplanted ones.
vertex only ? how about receding temples/hairline ?
We only tested on the vertex in the clinical studies, but people who used the sample products for their own testing say it also works for hairline recession.
Dear Jay,
So when you say “must regrow your lost hair based on our studies both in clinical and non-clinical” . Then am I correct to say the effect should be clear in before and after photos for regrowth in previously bald patch areas?
i.e. In your your new clinical trials.
The new clinical studies are still ongoing and we cannot say the outcome yet. We have some evidence though that the product works via molecular level triggering some important genes in hair shaft regeneration. Please see the 2017 paper from UT at http://genesdev.cshlp.org/content/31/8/744.full
Thanks Jay,
James
What happened to this?
On its way
Sorry but I can not find any information regarding this product from their website, medi-post.com. Is this product true ?
Kira, thanks for reading. The link is right in the article ? If by chance, there is some reason that you do not believe it there is not much I can do for that. Best wishes
Is the product just for females ?
Nope, for men and women 🙂
@admin,
There seems to have an extract of power point presentation in your post, would you mind sharing the full power point slide ? Many thanks
https://i0.wp.com/www.folliclethought.com/wp-content/uploads/2018/05/medipostdata.png
Hi, the only other slide is a title page.
Kira I have received additional information I will share through the blog soon. Thanks for your question.
thumbs up admin. waiting for your good news.
Is there any new update on medipost cm3???
Is there any new update on when it is releasing
Did the researchers who did the study test to see what happens if patients use it two times a day instead of one time per day? Also did the researchers test to see what happens if it’s used longer than 16 week? The product might be better if it’s used twice per day and it might be better if it’s used longer.
Hi Russel, a bigger scale clinical trial is ongoing with a protocol of being 2X per day posology for 24 weeks. The results will be available after Sep.https://clinicaltrials.gov/ct2/show/NCT03676400?term=medipost&rank=3
NGF-574H HAIR TONIC EARLY BIRD EXPERIENCE PROGRAM
Recently I received many inquiries asking how oversea customers order the product. We are going to set up a website for online direct purchase after official product launch in Korea at the end of this month. However, there are still many who cannot wait.
I am happy to announce that Celino decided to open an early bird product experience program for oversea customers to try NGF-574H hair tonic before the online website with early adopter benefits.
Please review attached form, fill out and send to join this special event. Product amounts are limited for this event. Thanks for your enthusiasm and support.
https://forms.gle/Kj6duTKsmN5ybPgH7