Featured Articles
The Best News in The Industry
An overview of treatments for pattern baldness and alopecia which will be in the clinical trial stage during 2023. Androgenetic Alopecia Trials Chong Kun Dang Pharmaceutical – We begin the list with a newcomer, previously unannounced. CKD-843 is a new injectable drug being trialed in “alopecia” by the Chong Kun Dang (CKD) Pharmaceutical company of Seoul, South Korea. Interestingly, the CKD-843 candidate is not yet listed on CKD Pharmaceutical’s pipeline page, however, we can gather some information from CKD-843’s clinical trial listing. The phase 1 trial testing for safety, pharmacokinetics, etc. indicates that there is some new/novel substance involved. The disease condition is vaguely listed as alopecia, yet the inclusion criteria mentions “those diagnosed with androgenic alopecia”, and the exclusion criteria mentions “alopecia areata”; thus, we can be sure this is a treatment for pattern hair loss or AGA. Finally, the administration method is noted to be through injection. Could this indicate that CKD-843 is one of Inventage Lab’s long lasting nanoparticle injections? Interestingly, Inventage Lab and CKD Pharmaceutical just announced a joint collaboration for an injectable dementia therapy. Yet, the Inventage Lab pipeline shows that its finasteride injections are partnered with Daewoong and Withus Pharmaceutical, only. This may further point…
Read MoreIn this article we will cover the most prominent therapeutic candidates in the alopecia areata treatment industry in 2023. Frontrunners To Market In June 2022, Eli Lilly/Incyte received the FDA’s first ever approval for a systemic treatment for alopecia areata with their drug Olumiant. Alopecia areata, an auto-immune disorder which results in patchy and sometimes total hair loss, differs from “common” hair loss or pattern baldness which is driven by androgen sensitivity. Prior to this FDA approval designation, baricitinib was previously an FDA approved drug for other conditions and was prescribed off-label for AA. Just days ago, Pfizer announced that its candidate for alopecia areata which has received “breakthrough” designation by the FDA, , ritlecitinib, met its endpoints in a phase 2b/3 study. Because of the breakthrough designation, Pfizer will be able to use data from the phase 2b/3 and from an ongoing long term study of ritlecitinib to file for FDA approval in the near future. Concert Pharmaceuticals has also reached a phase 3 trial for AA. Alopecia Areata Pharmaceutical & Biotech Therapies In Development AnaptysBio – Rosnilimab or ANB030 is a Anti-PD-1 agonist. It is currently being evaluated in a 45 patient phase 2 clinical trial in moderate…
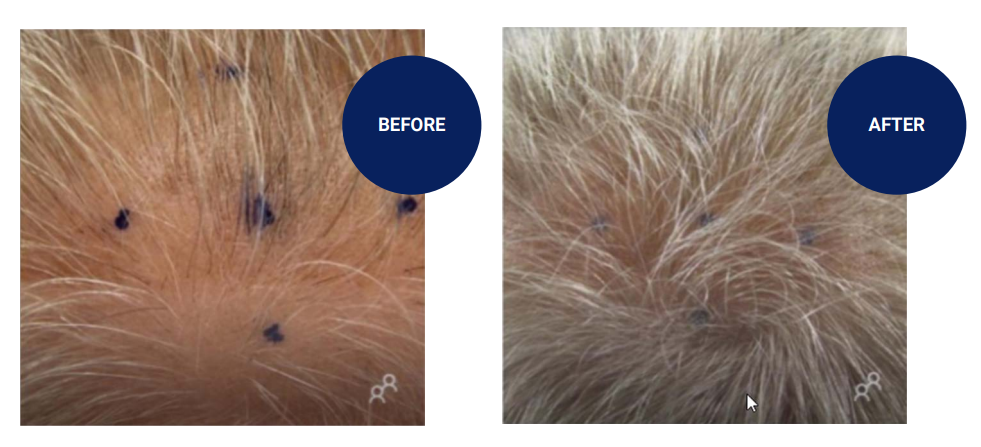
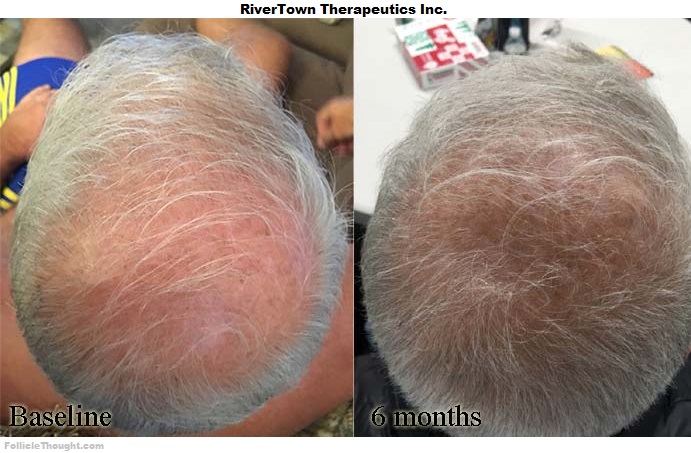
Read MoreUpdated October 2023 This post contains a list of the most viable and relevant hair regeneration treatments which are in development. They can be referred to as hair loss cures, baldness cures, hair growth treatments, hair loss treatments, or cures for androgenic alopecia. The treatments listed in this guide are ranked by a combination of their estimated hair growth potential and estimated release date to the public market. Ranking #1 is the most relevant and so on. Rankings on the list are always subject to change depending on information that becomes available. Directly below is a pipeline chart of the most clinically developed therapies in the hair growth industry, plus a few selected promising therapies. The estimated launch dates range from a “best case scenario” to a likely scenario. Naturally, all estimated launch dates are contingent upon the treatment passing efficacy and safety in clinical trials. 1. Kintor Pharmaceutical – Kintor currently owns two of the more interesting AGA therapeutic candidates within their pipeline, pyrilutamide and GT20029. Pyrilutamide is a topical androgen receptor antagonist which is being trialed in both androgenic alopecia and acne by Kintor. This would put it in the same category as the drug Breezula. A published…
Read MoreFor A List Of All Recent Articles Click Here
As Seen In

The New Yorker

InStyle

Trends in Biotechnology
Connect with Us
Let's Talk, Socially



Great site
Notify me of new posts.
Hello Marthinus,
Thanks for your comments, I really appreciate it. You may subscribe to new blog posts by entering your email in the right hand sidebar.
Cheers
Follicle Thought
very interesting site and gives hope aswell,
Thank you. Keep it up.
Thanks for the articles!
You’re welcome Jim!
Thank you very much for your hard work.Your article is very helpful .Best wishes from china.
Very deep thought! all your articles is worth reading speacially your Ultimate Guide to Hair Regeneration.
keep up the good work
Thanks for all the research, articles and all the updates. Best site I have found in the web.
Greetings from Germany
I really appreciate it Rene, thank you.
Hi Joseph, Thank you for taking the time and effort to share your research and thoughts on the progress of hair restoration. I really appreciate that you cover a wide range of systems without a biased opinion. I will definitely be following your site closely. Hopefully we are close to a cure! Best wishes
Thanks Steph 🙂
Hi .. Thanks for the site and info.
My hair is really thinning and I have been using rogaine , alas I see no real result. I am very disheartened because over the years I have lost my self confidence and rarely socialize. I know people have worst diseases like cancer and worst .., Is it too much to hope for a cure in a year or so .. I keep reading about new ways and drugs .. but I really don’t see how and when anything can become available in US. I am vain I suppose , guilty as charged , but if I don;t get my hair back I might die of loneliness that would lead to cancer and host of other diseases .. ANY NEW UPDATES? Please post something new.
Hi Matt,
I’m doing my best. There should be some good announcements over the next 3 months. Hang in there.
Joseph
Great research! Thank for helping us understand what’s up and coming.
Keep me updated.
Are you on Twitter for the sake of linking your articles? If not, you should be.
Ron,
Thank you very much! I am in fact, https://twitter.com/FollicleThought
Very interesting articles on your website. Your articles do give impression hairless will be successfully treated in coming years. But we are still waiting for news that something works here and now! Let’s hope we won’t all be too old if that day arrives)
This is a very helpful site
Hi Joseph, thanks for the well researched & honest website you have put together. Just touching base on the progress of the Turkish hair cream. Is it available anywhere to buy yet? Last I heard it was getting released to the Turkish market earlier in the year. I haven’t heard much since then. Any thoughts would be much appreciated.
Regards, Mick
Hi Mick,
Thank you for the kind words. Are you referring to Kelopesia? The last I’ve heard if that they are looking at a Jan 2017 release date or so. It has seemingly been a long time since we first heard the news, but we will have to wait for that date unless they announce some news.
Best,
Joseph
Thank you so much for this site.
Has anyone used/know of this product?
Morr-F Topical (Minoxidil 5% Finasteride 0.1%) from India
Great site!
Thank you for the updates. Appreciate it.
This site gives me hope. Tried Rogaine but ended up getting panic attacks and weird heart palpitations after a year. The worst panic attack was during a morning hangover after a night of bindge drinking. After that I called it quits on the rogaine. Rogaine is a vascular relaxer, that probably had something to do with the attacks and palpitations. I used to have lucious hair. I used to see balding men and feel bad and think I would never be like that. Now here I am in my twenties thinning out hard! Hoping a company like Replicell will create an affordable solution for this before I go completely bald. This is a helpful site.
Glad to hear the site is helpful to you Marty. Wish you the best.
Joseph,
Once this site had a very organized overview of updates listed out by company. Is that link or page still up? I realize most of the articles are updates but this was one overview odf the updates. Thanks
Right sidebar my friend
Hi Joseph just to reiterate our gratitude for follicle thought just thought I would pass on this link which to me looks very promising especially as its come from George Costeralis the godfather of hair research and will be
http://www.dailymail.co.uk/health/article-4483160/Breakthrough-spell-cure-baldness-grey-hair.html#v-1521587680001of interest to many
Hi Did you ever hear of a drug for AU called sulfasalazine? and what do you know about it?
Hi Ben,
Sorry I’ve never heard of it.
Very Inspiring site… Thank you for all your hard work. Had no idea there was so much new potential treatments in the pipeline. Brilliant news.
Thank you
i am writing this as a last hope i am 28 years age since 14 years i am facing apolecia areata . Dermatologists tried their best but all in vain .Get benefit for temporary basis hairs gets fine but after an year or few months its again vanish patch appears and then gets bigger and bigger leads towards Baldness. please help me ANything CAN PRP or transplant will help ? i want to get married etc
Have you researched JAK inhibitors for Areata or talked to your dermatologist about it?
I would like to get an update
Hi Bernabe, is there anything in particular you are looking for?
Hi – I have a couple of friends who have tried mesotherapy for their hair and have had astounding results – a full head of hair within 4-5 months! I haven’t tried it as I’m unsure what kind of side effects there are (haven’t seen any on my friends yet though). No one on this forum seems to recommend it or are talking about it. Is there a reason for that? Is it unsafe? Why aren’t more people advocating it? Thanks!
Hi Priya, the only thing I can think of is the results must not be seen in all of the patients who try it, otherwise it would naturally be known throughout the internet and hair industry. Any doctor’s office who created an effective mesotherapy treatment would have no problem spreading the word if it was truly producing results repeatedly. Photos of multiple patients would be necessary to corroborate the treatment’s efficacy.
Please notify me of new posts
Email subscription signup form is on the right sidebar and if you bookmark https://folliclethought.com/updates/ in your browser you will notice updates there.
Hi Joseph
There really seems to be a lot going on regarding the cure for hair restoration these days but it does seem a minefield out there of reliable information but most definatly the stem cell road seems to be the most conclusive for me but I came across this 2015 youTube clip on national USA TV from a Dr Harold Bafitis claiming he had a stem cell cure well worth a look and maybe worth tracking him down for any further info 3 years further down the line !
https://www.bing.com/videos/search?q=latest+baldness+news&&view=detail&mid=A18ED5D4E5D70D035369A18ED5D4E5D70D035369&&FORM=VRDGAR
Thanks for all your dedicated work Joseph
Thanks for your great website. I come here every week and pray that this week is the week that our prayers come true.
That is awesome to hear Jimmy, thanks very much.
Hi, I would like to thank you for your hard work! My daughter developed alopecia areata 1 year ago and it is hard for me as a mother to deal with it. Your site made me to believe that the cure/ therapy is just around the corner and helps me to live with hope. Thank you for structuring the data in such a neat manner and regular up-dates! There is no other place on the net like this. Keep up the good work!!!
Thanks for the kind note
Any news about WAY-316606?
Unfortunately, not. I am sure the research team is pursuing the development of the drug and maybe talking to company’s about doing a trial. But they have not released any news to the press about it. It may be months before we hear anything else.
Thank you for the great updated. Your page is one of the most important things keeping the hope for me. I am only 25 and having an alopecia one year ago. Currently, I am just starting to take finasteride 1mg everyday for almost one month in hope that someday there will be a way to cure baldness. From going through the documentation, I would like to know your opinion regarding to RCH-01 and Bronzo lotion whether will these product be a possibility to breakthrough this problem? I understand that a lot of people considered not taking finasteride because of it’s nasty effect and a lifetime taking in order to keep hair enacted. Since I just start to take finasteride, my idea is to still maintain my hair dermal in hope that cureness will be there soon. Thus, having your kind idea and update would be appreciated. Thank you and looking forward for your great updated as always.
They are certainly a possibility for breakthrough, but we will only know for sure when they are released.
Really appreciate the good work being done on this site. I am always following hair regeneration news, so this will be a huge aid for me. Many thanks.
Any updates on brotzu lotion (trinov)? I read it would be available in December? Will it be posted to Australia?
Bart, please read through the two latest articles on Trinov including the comments, the information is there.
I am wondering why there is no information on Kerastem? from their website it appears they are commercial in Europe and Japan. The press release from 2017 appears to involve a 17% increase in hair growth? seems promising! Perhaps I missed it when searching on the site here, but I’m surprised it’s not featured on the Ultimate Guide to Hair Regeneration 2018 list..
If you find any user reviews on this therapy let me know.
Can anyone recommend a hair transplant doc in Washington DC? Any thoughts on dr Lindsey? Thanks
Great site! This is such a great service you do for so many people. This site has gotten me through some of my worst days with this horrible process. Thank you so much, FollThoughts.
Btw, do you know if Tsuji’s human trials have started yet?
Dr Tsuji has cured hair loss they are saying in a video from Japan but the only problem is that it will cost over $100k that’s crazy
Hi Michael, can you repost this in the latest Aclaris article please?
I have been an avid reader of FT almost since inception and I congratulate you on your ability to follow the current developments in this field and keep your readers up to date. Unfortunately, from the time I began reading FT to today, the “promise” of a cure seems as elusive and remote today as in the beginning. Who knows, maybe the magic serum is right around the corner, or maybe hair cloning will become a reality some time soon. Or maybe five years from know we are all still going to be in this same waiting game. In the meantime, I have been going over past “potential cures” (who remembers sandalore from long ago 2019?) and came across the rosemary oil studies. Bottom line is that rosemary oil appears to be no less effective than minoxidil (I know, damning with faint praise) without the side effects. However, I cannot find any information that definitively addresses how rosemary oil should be applied in order to be most effective. Do you, or any of your readers, have any insight regarding the proper use of rosemary oil? Thanks again for all of your hard work.
Hi Mike, thanks for supporting Follicle Thought and for the congrats. Sorry to hear you’re not excited at all of the new developments over the past 2 years. Stemson, J Hewitt, Stemore, and many others are very exciting developments and I think the industry is the healthiest it’s ever been.
Regarding rosemary oil I don’t know if you will be able to find an effective way to use it on your hair. The reason I say this is because you’ll need to put the concentrated rosemary oil into a carrier oil (e.g. olive oil, almond oil, etc) and it will obviously leave hair greasy. I’m not sure if it’s really worth the while but perhaps you can do some digging on the net and find a pre-made product that is useful. I hope this helps.
Please provide details about the product RevivHair Max Serum.
I have heard that it works better than minoxidil and doesn’t have any side effect.
Moreover it does not have a tolerance effect like minoxidil.
Sam, I don’t believe there is a scientific basis to the claim you stated, if there is I will be hugely surprised.
@Follicle Thought Just bookmarked the updates page!! Great information to keep us on top of what is going on all in a single place. Tons of thanks!!
Thank you Dee.
Hi Folliclethought, I have found your website recently. I am a female in the mid thirties and have been diagnosed with AGA recently. I have probably lost 30% of my hair in 3-4 months. I’ve been on minoxidil for 5 weeks, saw palmetto and a topical estrogen DHT blocker as well as BC but I also want to get pregnant within the next 2 years which means I’ll have to get off all those medications. Are there any treatments coming in the next months / years that are promising for females / diffuse thinners that might be able to grow new hair and could be used during pregnancy? Thank you
Hi Holly, there are some new treatments coming in the next few years such as Breezula, SM04554, and even Histogen HSC, however it’s not clear if they can be used during pregnancy at this time.
Thank you
You forgot Follicum, they have a very promising solution for females
I was wondering what anyone thought of Nanoxidil. The company seems a bit odd, not so transparent and unusual. But I really wanted to know the results. Has anyone here used it? Lately it has become difficult finding real reviews because everyone has an affiliate link on their post or website making their opinions and views tainted in my opinion. I’ve lost a lot of hair lately I think due to COVID and stress and I don’t think it’s coming back unless I do something. Thank you
Hi everyone, does anyone have experience with the Nutra M hair serum (topical DHT blocker) by Advanced Trichology? I am wondering what I can use as a woman to block DHT in the scalp. Thank you!
Hi Holly, I don’t have any experience with it but it may have a mild positive effect. Melatonin has shown to have a positive effect on hair growth.
Do you apply the melatonin directly to the scalp or take it orally? I know you can get the pills at the drug store. How much does one need to take, and how often, for visible results?
Thanks.
Hi
Do you have any update on the results of the Histogen 1b/a study?
All material I have studied about Gail Naughtons erlier studies and patents tells me this study will be a success.
Ok I saw now the part about updates..
I still wonder what your opinion is about Histogens latest trial, and technology.
I’ve got good hopes for Histogen’s latest trial actually, they’ve shown numbers in the past this time they are on the primetime stage, need to see successful numbers and HSC will be acquired by a larger partner no doubt.
I am a 48 yo female with Andro Alopecia and my doctor just prescribed oral minoxidil. I’m starting out on 1.25mg and hopefully progressing to 2.5mg’s. I am still using the topical 5% until further notice from the dr. I am extremely concerned that switching will worsen my condition. Do you have any feedback or knowledge on oral minoxidil as well as making the transition?
Hi JM, I don’t have any personal experience in taking oral minoxidil but in general I have heard that people tend to see a bit better results with oral minoxidil. If you keep observing your hair with your doctor’s supervision you should be doing good.
I have heard much about Reviv-Hair Serum that it actually works better than minoxidil. It has many ingredients. Please @Follical thought if you can do some research and about the same.
I will look Aditya.
I am a woman in my early 60’s, but look young for my age. My hair has been noticeably thinning, especially on the sides of my head by my face for a year or so. I have been waiting for one of the JAK inhibitor drugs to come on the market in products like shampoos or even a safe pill form, but am unable to find out if and when any such products or pills might be safely sold to the general public (or through a doctor). I have had hair grafts, but they are thinning as well, so I have had some of the hair strands micro bladed into my scalp to hide the places that are thinning. However, if someone gets near me and looks at it up close, it doesn’t look natural. What is the latest news on JAK inhibitor products and when do the scientists think it will become available to the general public? Thanks you.
Hi Deedee, for common hair thinning and not alopecia areata JAK inhibitor drugs are not ideal. The drugs already exist on the market as approved prescription medications and you can get them compounded into topical formulas. However, this is only typically done for alopecia areata patients. Have you seen a dermatologist about hair growth drug options? I recently did an article about topical finasteride companies and one of them, Strut Health, offers a formula for women you could review.
No more promising companies in phase 2?
Kintor is most promising now.
I have been visiting the site since 2017 and I see the chart every year. Two years later, for example, 2017 hit 2018-2020.
I think the problem is not the cure, but the corona treatment was more complicated than the fall, and they made the rna vaccine. But I think the hair has more material aspect because with its treatment, the skin and hair doctor loses their income.
Hi
You have missed the surprising news from Follicum yesterday. The game is not over for them.
Hi thanks for sharing Jens. I will look into it.
Did you look into it?
Regards
Jens
Yes I did Jens, it seems the CEO still said “no one on our team expects that the new analysis is going to change anything.” It seems their hair program is still completed.
Fisetin promotes hair growth in mice:
https://www.mdpi.com/2072-6643/13/6/2087
https://www.frontiersin.org/articles/10.3389/fcell.2020.566617/full
Really appreciate this site, and all the hard work that must go into it. I understand that the focus is on monitoring cures/research – is there a place you’d recommend for best practices on hair restoration, using what we have now?
Thanks Mick, I would say that reddit Tressless and other message boards dedicated to hair transplant surgery are the best options right now.
Awesome, thanks admin. As an aside – how do you read the current drama with replicel and shiseido? I’ve read that replicel claimed it didn’t have the resources to perform trials, but it could be business speak for, the product isn’t ready, or might not have wings at all, etc. What’s your take?
I mean, I don’t think Replicel had the money for the trials, but that’s just my opinion.
I am think do some prp of dutasteride this year. I will only this, no minoxidil and finasteride. is really effective and with 3 season the year is will?
well *
I would like to ask about (BLUE HAIR CARE SERUM) medication
And how effective is it for genetic baldness?
I never heard of it addusalam, I see now its ingredients are OK. It seems like it would be mediocre.
Hi Admin
Do you have connections to hair surgeons and hair researchers who would be able to comment on Dr Hasson’s comments regarding premature facial aging from minoxidil? I have noticed facial sagging since starting minoxidil but am not 100% sure if it is due to the minoxidil. My dilemma is that minoxidil is doing wonders for my hair, so I really don’t want to discontinue it without confirmation that it is causing premature aging. Ultimately I would choose my face over my hair.
Thanks for your insight.
I would like to ask about (BIO HAIR SERUM) medication
And how effective is it for genetic baldness?